The anode material in almost all of today’s secondary lithium-ion batteries is graphite. In order to boost the energy density, we would love to replace the graphite by lithium metal. Many people work on this goal. Unfortunately, the lithium metal anode tends to form dendrites during electrochemical plating, eventually... read more →
Dec
18
Oct
12
In a previous application note named “1001 reasons for using a reference electrode” we tried to convince you of the advantages of three-electrode cells with reference electrode over so-called half cells with lithium metal counter electrode. We claimed: “The large overvoltage at the lithium metal CE, the chemical reactivity of... read more →
Jul
10
The FS-5P is a double-layered separator comprised of a 180 µm thick nonvowen PP cloth (Freudenberg FS 2226 E) and a 38 µm thick microporous UHMW-PE membrane (Lydall Solupor 5P09B). We offer PAT insulation sleeves with this FS-5P separator, either with or without a lithium reference. When built into the... read more →
Jul
07
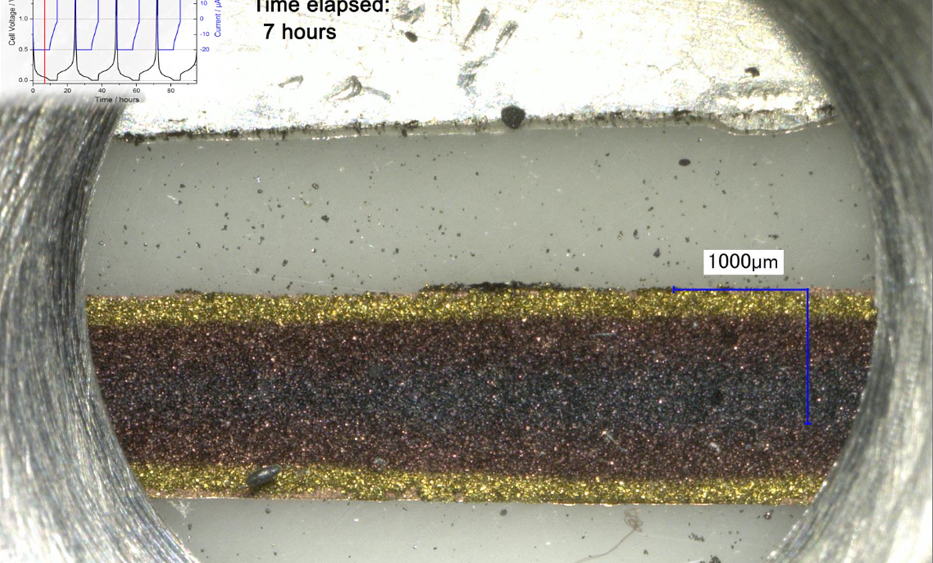
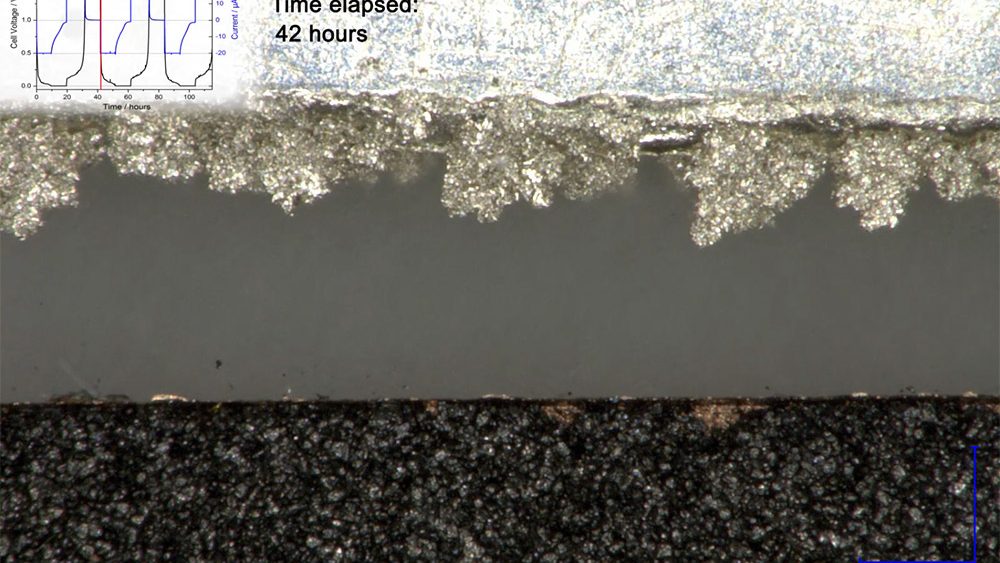
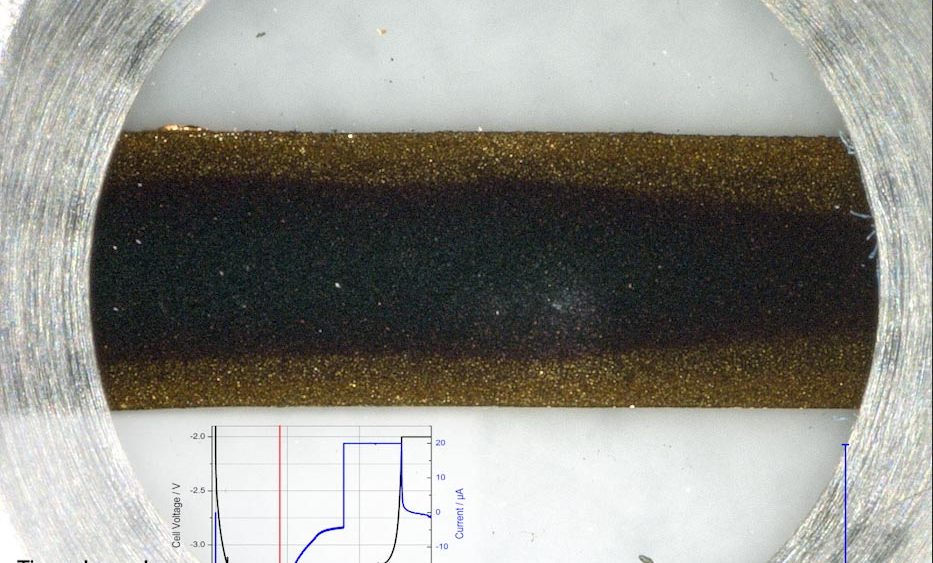
Former test setup In a previous report, we have used the ECC-Opto-Std test cell to visualize the electrochemical lithiation of a free-standing graphite electrode sandwiched with a lithium metal counter electrode as the lithium source. Sketch 1a shows the sandwich geometry of the set-up used at that time. Notably, the... read more →
Jun
02
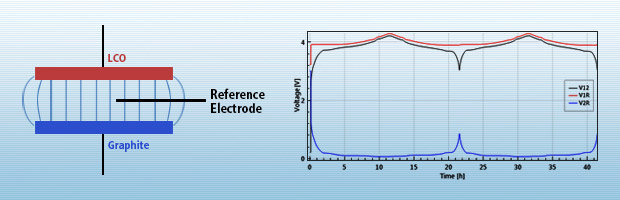
There are many good reasons for using a reference electrode. In this note we report a few of them using the example of a lithium-ion battery comprising a lithium cobalt oxide (LCO) cathode and a graphite anode. All measurements were performed with a PAT-Cell, docked into a PAT-Stand-16 (both EL-Cell).... read more →
Jul
01
Because of the outstanding reliability of the built-in lithium metal reference electrode, the PAT-Cell is the ideal test cell for long-term 3-electrode experiments on Li-ion battery systems. The BioLogic VSP potentiostat, on the other hand, is a perfect match for controlling this type of experiment, as it is capable of monitoring... read more →