
In our last application note we took a closer look at the cathode “half-cell” of the lithium-ion battery, which in our case was NCM against lithium metal. It turned out, that the lithium metal electrode is under some conditions the bottleneck of charge transfer, especially at the vertex points of the charge-discharge cycle, and that a reference electrode is indispensable to understand this effect.
In the present note, we extend our investigations to the anode “half-cell”, which is graphite against lithium metal. Again, we built a PAT-Cell using a glass fiber separator and a standard LiPF6 based electrolyte. An insulation sleeve with built-in lithium metal reference was used in order to monitor the single electrode potentials during the experiment.
—

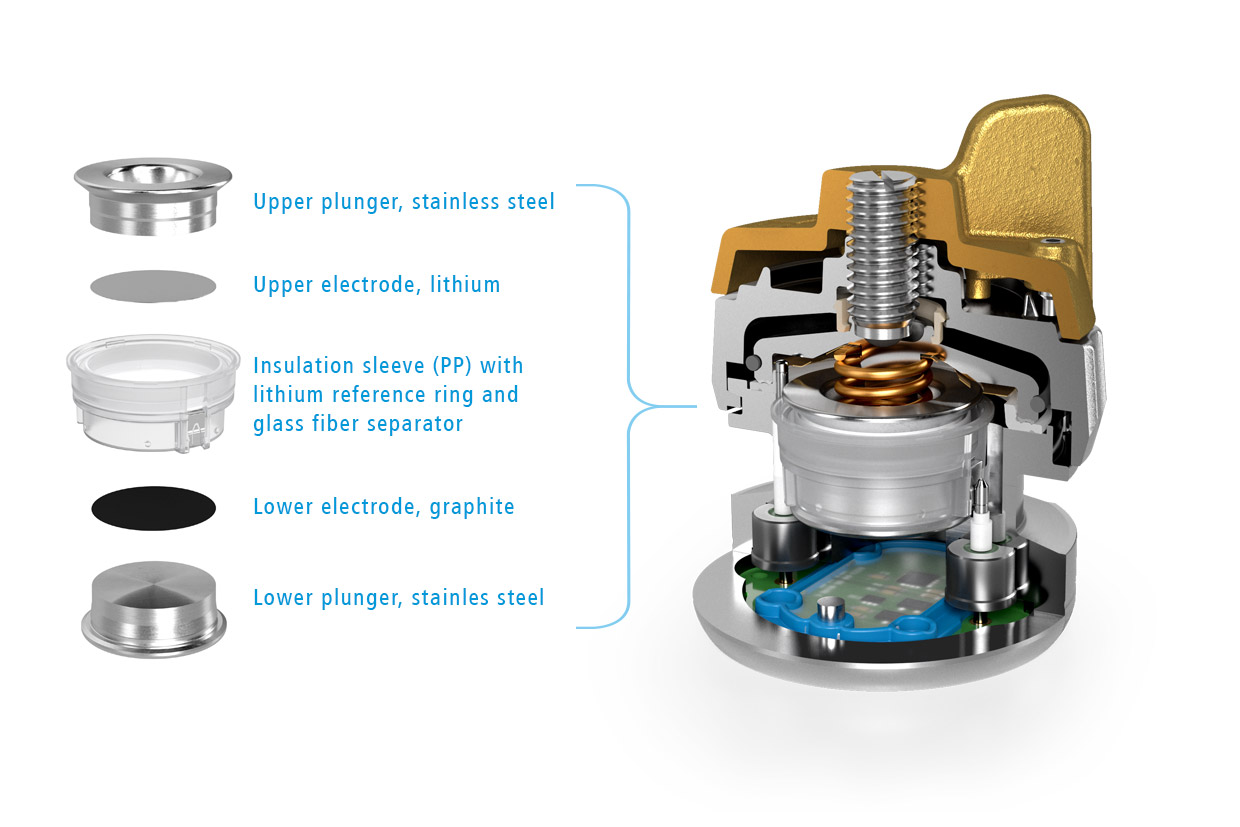
Figure 1: Sketch showing the cell stack of the Graphite-Li-Li(R) cell. Graphite was used as the lower electrode (1), lithium metal for both the upper electrode (2) and the ring-shaped reference electrode (R).
Video 1: Assembling the PAT-Cell:
The test procedure is to cycle the graphite electrode with a constant current of 1 mA between V1R = 2.5 and 0.005 V. Intermittently, every 5 minutes, a small sinusoidal current is superimposed on the direct current at frequencies between 10 kHz and 1 Hz to measure the impedance.
Video 2: EL-Software – Writing the test procedure
Video 3: EL-Software – Running the experiment
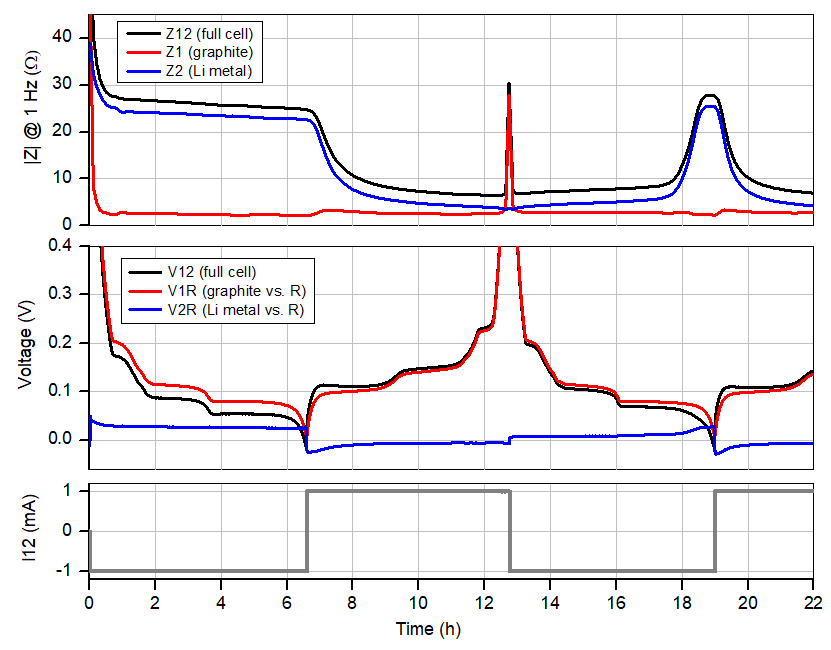
Figure 2: Cell current, voltages and impedances at 1 Hz of the graphite-Li-Li(R) PAT-Cell. The cycle starts at a (rest) cell voltage of 2.8 V. Only the “more exciting” voltage range <0.4V is shown. Note that the Li metal electrode is always the “slower” (i.e., higher Z) electrode except when the graphite is fully delithiated.
The diagrams in figure 2 show from bottom to top the applied current i12, the voltages (V12, V1R, V2R) and the impedances (magnitudes of Z12, Z1, Z2 at 1 Hz) of the full cell and the individual electrodes.
What do we observe ?
Notably, the cycle of the anode half-cell has to start with negative current (discharge) so as to mimic what happens at the graphite electrode in the “real” Li-ion battery (NCM vs. Graphite) during first charge. Consequently, at the beginning of the cycle, the lithium metal electrode (2) is getting dissolved, and lithium ions are getting inserted into the graphite electrode (1). The dissolution of lithium metal leaves behind a smooth (i.e. low surface area) electrode covered with the SEI layer and consequently the impedance Z2 of the lithium electrode remains high throughout the initial half cycle. This situation changes with the reversal of the current direction after 6.2 hours. Now lithium ions are extracted from the graphite lattice and deposited as a porous layer of dendritic metal on the lithium metal electrode. This increase in surface area leads to the observed decrease of |Z2| in the second half cycle.
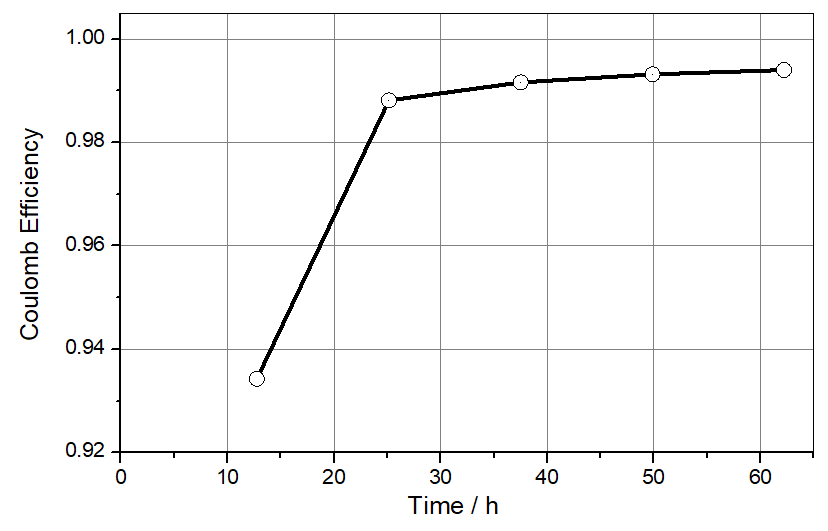
The Coulombic efficiency for the graphite lithiation/de-lithiation is found to be 93% in the first cycle and >99% in the consecutive cycles (see Figure 3). In contrast, the Coulombic efficiency for the deposition/dissolution of lithium metal never exceeds 95% (taking that number from our previous application note for the plating on stainless steel). The porous and thus low-impedance lithium metal deposited during a given half cycle has thus been fully consumed in the next half cycle when the graphite electrode is still about 5% away from full lithiation. This last 5% must be dug out of the original non-porous (and therefore high-impedance) lithium metal foil, resulting in the observed rise in the impedance Z2. This behavior is seen for the first time after 18 hours and then during each consecutive cycle. We saw and discussed the same effect in the last application note for the NCM half-cell.
Again, the lithium metal is lost by electrical disconnection of lithium metal pockets during dendrite dissolution. The disconnected but still metallic pockets can be observed as a greyish residue in the separator and on the surface of the lithium metal electrode when dismantling the PAT-Cell after the cycle experiment.
Video 4: Disassembling the PAT-Cell
What can we conclude?
- Graphite against lithium metal is not only a poor battery in that it stores almost no energy, but, perhaps more surprisingly, it is also a poor experimental model for the investigation of the graphite anode in the lithium-ion battery.
- Almost all the time during the cycle experiment, the lithium metal electrode rather than the graphite electrode is the bottleneck for charge transport. Especially in the initial half-cycle and then always at the end of graphite lithiation, when the cell voltage is close to zero, the high overvoltage at the lithium metal electrode leads to a premature termination of the cycle and thus to an incorrect (too small) capacity. Due to the flat voltage profile, this is much more problematic with the graphite half-cell than it was with the NCM half-cell.
- The high overvoltage at the lithium metal electrode can be corrected for by controlling the graphite potential V1R rather than the full cell voltage V12. We did so in our experiment taking advantage of the reference electrode. Even then, however, lithium dendrites can prematurely end the life of the cell. The porous layer of “dead” lithium absorbs electrolyte solution, causing the cell to dry out and lose capacity. And also the soluble reaction products of the intensive SEI formation on the lithium metal can unpredictably affect the performance of the cell. All those effects will not be seen in the Li-ion battery.
Our advice:
Build your test cells with the anodes and cathodes that are in the real battery. Only use a lithium metal anode if you want it to be in the final battery. And whether you work with or without a lithium metal anode, always use a reference electrode as a third electrode. Whenever possible. Did I mention that already in the previous app note?
Next time we will finally deal with the real lithium ion battery made of NCM and graphite. Completely without lithium metal, except, of course, for the reference electrode.
Stay tuned (and healthy).
— by Dr. Matthias Hahn, Dr. Annika Baumann, Margaryta Paramonova, Daniel Wilke



Comments are closed.