New cathode and anode materials for lithium ion batteries (LiB) are often tested for their electrochemical performance using lithium metal as the counter electrode. In laboratory language, these configurations are sometimes called “half-cells”. In most cases no reference electrode is used for such half-cells because the electrode potential of the lithium metal electrode is considered to be pinned to 0 V vs. Li/Li+. The half-cell concept is impressively simple and has proven itself for many questions. However, we will show here that half-cell experiments have their limits.
In this note we will first take a closer look at the cathode half-cell. For that purpose we have built a PAT-Cell with NCM 111 against lithium metal using a glass fiber separator and a standard LiPF6 based electrolyte. An insulation sleeve with built-in lithium metal reference was used in order to monitor the single electrode potentials during the experiment.
—

Figure 1b: Sketch showing the cell stack of the NCM-Li-Li(R) cell. NCM 111 was used as the lower electrode (1), lithium metal for both the upper electrode (2) and the ring-shaped reference electrode (R).
Video 1: Assembling the PAT-Cell:
The test procedure is to cycle the NCM half-cell with a constant current of 1 mA between 2.5 and 4.3 V cell voltage (V12). Intermittently, every 5 minutes, a small sinusoidal current is superimposed on the direct current at frequencies between 100 kHz and 0.1 Hz to measure the impedance. For the sake of simplicity, only the magnitude of the impedance at 0.1 Hz – the effective “DC resistance” – is shown and discussed below.
Video 2: EL-Software – Writing the test procedure
Video 3: EL-Software – Running the experiment
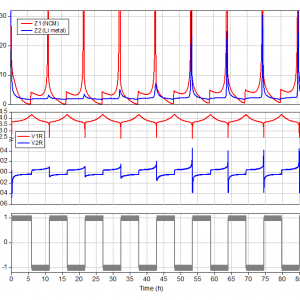
Figure 2: Cell current, voltages and impedance at 0.1 Hz of the NCM-Li-Li(R) PAT-Cell. Note that the voltage axis in diagram (b) is interrupted to emphasize the overvoltage at the lithium metal electrode.
The diagrams in figure 2 show from bottom to top the applied current i12, the voltages (V12, V1R, V2R) and the impedances (magnitudes of Z12, Z1, Z2 at 0.1 Hz) of the full cell and the individual electrodes.
What do we observe (and what do we speculate)?
By applying a positive current i12, lithium ions are getting extracted from the NCM electrode (1) and deposited (plated) at the lithium metal electrode (2). In this way, a porous layer of dendritic lithium metal grows on the original non-porous lithium surface. The increase in surface area is reflected in a decrease of overvoltage |V2R| and impedance |Z2| of the lithium metal electrode. When the current direction is reverted after 6 hours, the previously formed porous metal layer is dissolved at low overvoltage. The slight increase of |V2R| and |Z2| at the end of this discharge half cycle, after about 11 hours, indicates that the porous lithium layer is almost consumed at this point, so that non-porous lithium must be dissolved again. This effect becomes more and more pronounced in the following cycles.
A curious observation
A somewhat curious thing about the NCM half-cell is that at the very beginning lithium metal is deposited on lithium metal. Wouldn’t it be sufficient (and easier) if the lithium from the NCM was deposited on an inert metal sheet in the first cycle, and this lithium then recycled?
We have tested this idea by cycling NCM against a plain stainless steel plunger using the same test protocol as in the first experiment.

Figure 3a: Sketch showing the PAT-Core configuration (cell stack) with NCM 111 against stainless steel inside the PAT-Cell.
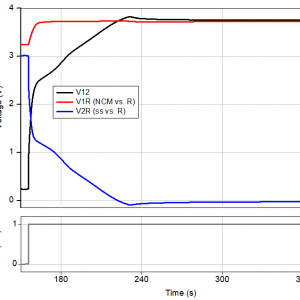
Figure 3b: The initial minutes of experiment 2. Plating lithium metal on the stainless steel plunger (ss).
As can be seen in figure 3b, it takes approximately 80 seconds to get the potential of the stainless steel plunger down to 0 V vs. Li/Li+. During the next 6 hours the plunger is plated with lithium. Then, until the end of the third cycle (labeled A in figure 4), the cell runs very similar to the first experiment. At this point, both V2R and |Z2| suddenly rise, indicating that the anode can no longer supply the required lithium to “refill” the NCM.
Where has the lithium gone?
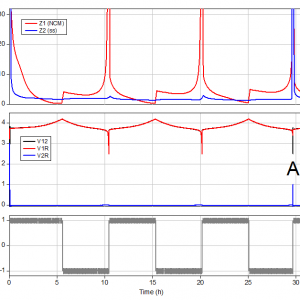
Figure 4: The initial 32 hours of experiment 2. After 29 hours (label A) the lithium supply on the stainless steel plunger is depleted. The end point of the 3rd cycle is thus determined by the rise of V2R and no longer by the fall of V1R.
The reason for this behavior can be understood when plotting the Coulomb efficiencies of the two experiments, figure 5. For both experiments, the Coulomb efficiency of the 1st cycle is 88%. That means that 12% of the lithium extracted during the first half cycle cannot be “refilled” into the NCM lattice during the second half cycle. Assuming that all the lithium extracted from the NCM would be plated on the stainless steel plunger (i.e. with 100% Coulomb efficiency), this amount of lithium would be sufficient for the subsequent cycles until the end of days, regardless of the Coulomb efficiency of the NCM electrode. Unfortunately, the Coulomb efficiency of lithium plating/stripping turns out to be only 95 %, while that of the lithium ion extraction/insertion into and out of NCM is already >99% in cycle 2. As a consequence, the stock of lithium metal built up in the first half cycle is already exhausted at the end of the third cycle.
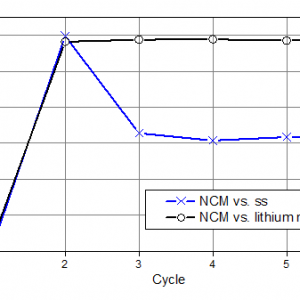
Figure 5: The evolution of the Coulomb efficiencies of the two experiments. In the NCM vs. stainless steel (ss) cell, the initially plated lithium metal is used up at the end of cycle 3, resulting in the observed drop of the Coulomb efficiency. In the NCM-Li cell, the large excess of lithium metal ensures that the charge efficiency is solely determined by the NCM electrode.
This in mind, we now understand why overvoltage and impedance of the lithium metal electrode increase at the vertex points of each cycle and why this effect is getting more pronounced after a few cycles (cf. figure 2). This is because some fresh, non-porous lithium metal must be dissolved at the end of each cycle in order to compensate for the lithium metal lost during this cycle. And so unfortunately, we cannot do without the lithium metal counter electrode in the NCM half cell. The excess of lithium metal is needed to compensate for the 5% loss during plating/stripping. Using Faraday’s law, we calculate that about 65 µg of lithium metal is lost per cycle in our experiments. (5% of 5 mAh / 96500 As mol-1 * 7 g/mol).
1 mg of excess lithium will keep our cell running for 150 hours.
The lithium metal is believed to be lost by chemical reaction with the electrolyte solution (and thus forming the SEI) and by electrical disconnection of metallic lithium (Li0) pockets during dendrite growth and dissolution. The disconnected but still metallic lithium can be observed as a greyish residue in the separator and on the surface of the lithium metal electrode when dismantling the fully discharged PAT-Cell after the experiment. Only recently it was reported that the inactive Li0 can be quantitatively determined as hydrogen gas by reaction with water [Ref 1]. The authors state that most lithium is lost by electrical disconnection (and so is still present in the form of disconnected lithium metal) and not by chemical reaction.
Video 4: Disassembling the PAT-Cell
What can we conclude?
- The plating/stripping of lithium metal is not fully reversible. In our case, in each cycle, about 5% of the plated lithium metal is lost either by chemical reaction with the electrolyte solution or by electrical separation of still metallic lithium from the “mother” anode.
- The lithium dendrites growing into the separator can cause a short circuit with the NCM cathode and thus limit the life of the cell. Thus, long-term cycling and aging experiments with a lithium metal anode is difficult.
- The impedance of the lithium metal anode changes significantly during each cycle and from cycle to cycle. It temporarily even dominates the kinetics of the cell, especially at the beginning of the first cycle and in the further course of the experiment always at the end of the discharge. Thus, the kinetics of the NCM electrode obviously cannot be determined in a two-electrode arrangement with a lithium metal anode without reference electrode. You should always know the impedance of both anode and cathode
Our advice:
Build your test cells with the anodes and cathodes that are actually in the finished battery. Only use a lithium metal anode if you want it to be in the finished battery. And whether you work with or without a lithium metal anode, always use a reference electrode as a third electrode. Whenever possible. Did I mention that you should use our PAT system?
In the next note of this series we will address the so-called anode half-cell, i.e. graphite against lithium. Maybe you can guess what’s going to happen. But there’s a surprise. Stay tuned (and healthy).
—
[Ref 1] Fang, C., Li, J., Zhang, M. et al. Quantifying inactive lithium in lithium metal batteries. Nature 572, 511–515 (2019).
by Dr. Matthias Hahn, Dr. Annika Baumann, Margaryta Paramonova, Daniel Wilke




Comments are closed.